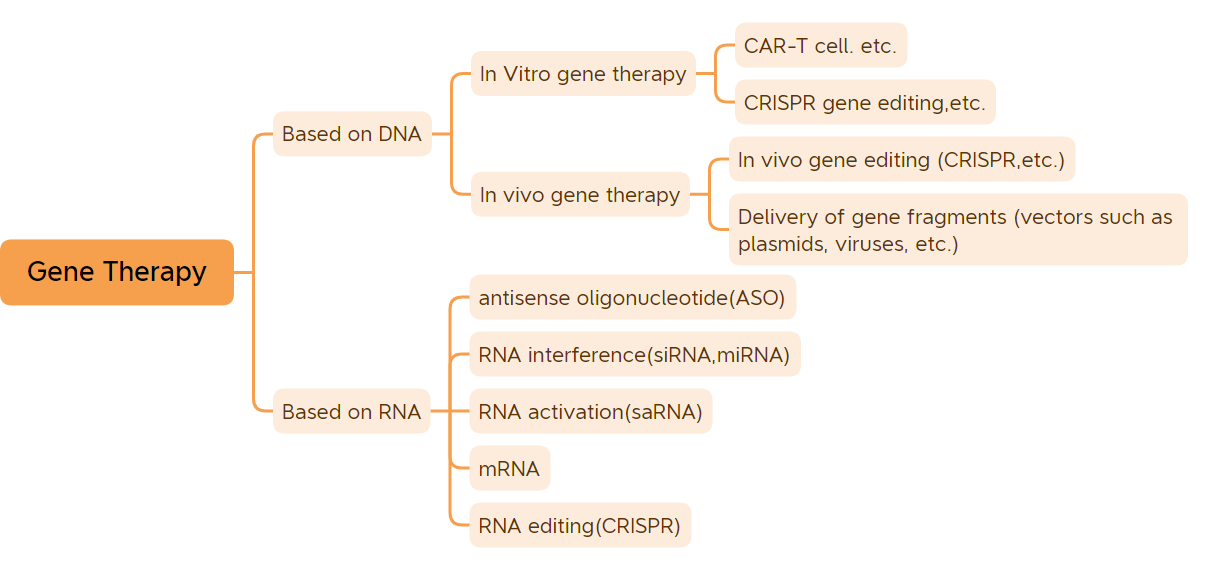
Mkpụrụ ndụ ihe nketa bụ ngalaba mkpụrụ ndụ ihe nketa na-achịkwa àgwà.Ewezuga mkpụrụ ndụ ihe nketa nke ụfọdụ nje, bụ́ ndị mejupụtara RNA, mkpụrụ ndụ ihe nketa nke ihe ka ọtụtụ n'ime ihe ndị dị ndụ bụ DNA mejupụtara..Ihe ka ọtụtụ n'ọrịa ndị dị ndụ na-ebute site na mmekọrịta dị n'etiti mkpụrụ ndụ ihe nketa na gburugburu ebe obibi.Usoro ọgwụgwọ mkpụrụ ndụ ihe nketa nwere ike ịgwọ ma ọ bụ belata ọtụtụ ọrịa.A na-ahụta ọgwụgwọ mkpụrụ ndụ ka ọ bụrụ mgbanwe n'ọhịa ọgwụ na ụlọ ahịa ọgwụ.Ọgwụ ọgwụgwọ mkpụrụ ndụ ihe nketa n'ụzọ sara mbara gụnyere dabere na ọgwụ DNA megharịrị DNA (dị ka ọgwụ ọgwụ vivo gene therapy dabere na vector vectors, in vitro gene therapy ọgwụ, plasmid ọgwụ gba ọtọ, wdg) na ọgwụ RNA (dị ka ọgwụ antisense oligonucleotide, ọgwụ siRNA, na ọgwụgwọ mkpụrụ ndụ mRNA, wdg);mmetụta dị warara ọgwụ ọgwụgwọ mkpụrụ ndụ gụnyere ọgwụ plasmid DNA, ọgwụ ọgwụgwọ mkpụrụ ndụ ihe nketa dabere na vectors viral, ọgwụ mkpụrụ ndụ ihe nketa dabere na vectors nje, sistemu mkpụrụ ndụ ihe nketa, na ọgwụ ọgwụgwọ mkpụrụ ndụ maka mgbanwe mkpụrụ ndụ in vitro.Ka ọtụtụ afọ nke mmepe siri ike gasịrị, ọgwụ ọgwụgwọ mkpụrụ ndụ ihe nketa enwetawo nsonaazụ na-agba ume na ụlọ ọgwụ.(Agụghị ọgwụ mgbochi DNA na ọgwụ mgbochi mRNA) Ugbu a, akwadola ọgwụ ọgwụgwọ mkpụrụ ndụ ihe nketa 45 maka ire ahịa n'ụwa.Ngụkọta nke usoro ọgwụgwọ mkpụrụ ndụ 9 ka akwadoro maka ire ahịa n'afọ a, gụnyere usoro ọgwụgwọ mkpụrụ ndụ asaa akwadoro maka ịzụ ahịa nke mbụ n'afọ a, ya bụ: CARVYKTI, Amvuttra, Upstaza, Roctavian, Hemgenix, Adstiladrin na Ebvallo, (Rịba ama: Abụọ ndị ọzọ ka akwadoro na United States, bụ nke a kwadoro ụdị nke mbụ nke ZFDA n'ahịa n'afọ a. maka ịre ahịa na United States n'August 2022, ndị European Union kwadoro maka ịre ahịa na 2019; .) Site na mwepụta nke ọtụtụ ngwaahịa ọgwụgwọ mkpụrụ ndụ ihe nketa na mmepe ngwa ngwa nke teknụzụ ọgwụgwọ mkpụrụ ndụ ihe nketa, ọgwụgwọ mkpụrụ ndụ ihe nketa na-achọ iweta oge mmepe ngwa ngwa.
Nhazi ọgwụgwọ mkpụrụ ndụ ihe nketa (isi mmalite foto: Bio-Matrix)
Edemede a depụtara usoro ọgwụgwọ mkpụrụ ndụ iri anọ na ise (ewezuga ọgwụ mgbochi DNA na ọgwụ mRNA) nke akwadoro maka ire ahịa.
1. In vitro gene therapy
(1) Strimvelis
Ụlọ ọrụ: GlaxoSmithKline (GSK) mebere ya.
Oge ịre ahịa: European Union kwadoro ya maka ire ahịa na Mee 2016.
Ngosipụta: Maka ọgwụgwọ nke nnukwu mkpokọta immunodeficiency (SCID).
Nkwupụta: Usoro ọgwụgwọ a na-emekarị bụ ibu ụzọ nweta mkpụrụ ndụ hematopoietic nke onye ọrịa, gbasaa ma mebe ha na vitro, wee jiri retrovirus webata mkpụrụ ndụ ADA (adenosine deaminase) na-arụ ọrụ n'ime mkpụrụ ndụ hematopoietic, n'ikpeazụkwa, a na-agbanyeghachi mkpụrụ ndụ hematopoietic gbanwetụrụ n'ime ahụ.Nsonaazụ ụlọọgwụ na-egosi na ọnụọgụ ndụ afọ 3 nke ndị ọrịa ADA-SCID ejiri Strimvelis gwọọ bụ 100%.
(2) Zalmoxis
Ụlọ ọrụ: Ụlọ ọrụ Italy MolMed mepụtara.
Oge ịre ahịa: Nweta ikike ịre ahịa ọnọdụ n'aka European Union na 2016.
Ngosipụta: A na-eji ya maka ọgwụgwọ adjuvant nke usoro ahụ ji alụso ọrịa ọgụ mgbe a gbasasịrị mkpụrụ ndụ hematopoietic stem cell.
Okwu: Zalmoxis bụ ihe allogeneic T cell igbu onwe mkpụrụ ndụ immunotherapy nke vectors retroviral gbanwere.Usoro a na-eji vectors retroviral iji gbanwee mkpụrụ ndụ allogeneic T mkpụrụ ndụ, nke mere na mkpụrụ ndụ T ndị a gbanwere na-egosipụta 1NGFR na HSV-TK Mut2 mkpụrụ ndụ igbu onwe ha na-enye ndị mmadụ ohere iji ọgwụ ganciclovir (ganciclovir) n'oge ọ bụla iji gbuo mkpụrụ ndụ T nke na-eme ka mmeghachi omume na-adịghị mma, gbochie ohere ọzọ na-emebi GVHD, ma na-enye ndị ọrịa na-ahụ maka ọrịa na-adịghị ala ala nke GVHD.
(3) Invossa-K
Ụlọ ọrụ: TissueGene mepụtara (KolonTissueGene).
Oge ịre ahịa: Ekwenyere maka ndepụta na South Korea na Julaị 2017.
Ngosipụta: Maka ọgwụgwọ ọrịa ogbu na nkwonkwo degenerative ikpere.
Nkọwa: Invossa-K bụ usoro ọgwụgwọ mkpụrụ ndụ ihe nketa allogeneic metụtara chondrocytes mmadụ.A na-emezi mkpụrụ ndụ allogeneic na mkpụrụ ndụ ihe nketa na vitro, mkpụrụ ndụ ndị a gbanwere nwere ike igosipụta ma zoo ihe na-agbanwe uto β1 (TGF-β1) mgbe ịgba ọgwụ mgbochi intra-articular gasịrị.β1), si otú ahụ na-emeziwanye ihe mgbaàmà nke ọrịa ogbu na nkwonkwo.Nsonaazụ ụlọ ọgwụ na-egosi na Invossa-K nwere ike melite ogbu na nkwonkwo ikpere.Ndị nchịkwa nri na ọgwụ ndị Korea wepụrụ ya na 2019 n'ihi na onye nrụpụta ahụ mejọrọ ihe eji eme ihe.
(4) Zynteglo
Ụlọ ọrụ: Achọpụtara ma mepụta ya site na bluebird bio.
Oge ịre ahịa: European Union kwadoro maka ịre ahịa na 2019, yana FDA kwadoro maka ịre ahịa na United States n'August 2022.
Ngosipụta: Maka ọgwụgwọ β-thalassemia na-adabere na mmịnye ọbara.
Nkọwa: Zynteglo bụ ọgwụ lentiviral in vitro gene therapy nke na-ewebata nnomi nke na-arụ ọrụ nke mkpụrụ ndụ β-globin nkịtị (βA-T87Q-globin gene) n'ime mkpụrụ ndụ hematopoietic nke e si n'aka onye ọrịa nweta site na vector lentiviral, wee nyeghachi mkpụrụ ndụ hematopoietic autologous hematopoietic a gbanwere n'ime onye ọrịa.Ozugbo onye ọrịa nwere mkpụrụ ndụ βA-T87Q-globin nkịtị, ha nwere ike imepụta protein HbAT87Q nkịtị, nke nwere ike belata nke ọma ma ọ bụ wepụ mkpa mmịnye ọbara.Ọ bụ ọgwụgwọ otu oge e mere iji dochie mmịnye ọbara ogologo ndụ na ọgwụ ndị na-adịru ogologo ndụ maka ndị ọrịa gbara afọ 12 gbagowe.
(5) Skysona
Ụlọ ọrụ: Achọpụtara ma mepụta ya site na bluebird bio.
Oge ịre ahịa: European Union kwadoro maka ịre ahịa na Julaị 2021, yana FDA kwadoro maka ịre ahịa na United States na Septemba 2022.
Ngosipụta: Maka ọgwụgwọ nke mmalite ụbụrụ adrenoleukodystrophy (CALD).
Okwu: Skysona gene therapy bụ naanị otu oge usoro ọgwụgwọ mkpụrụ ndụ ihe nketa akwadoro maka ọgwụgwọ nke ụbụrụ adrenoleukodystrophy nke mbụ (CALD).Skysona (elivaldogene autotemcel, Lenti-D) bụ hematopoietic stem cell lentiviral in vitro gene therapy Lenti-D.Usoro usoro ọgwụgwọ n'ozuzu bụ nke a: a na-ewepụ mkpụrụ ndụ hematopoietic autologous n'aka onye ọrịa, gbanwee ma gbanwee ya site na lentivirus na-ebu mmadụ ABCD1 gene in vitro, wee weghachite ya na onye ọrịa.A na-eji ya agwọ ndị ọrịa nọ n'okpuru afọ 18, na-ebu mmụgharị mkpụrụ ndụ ABCD1, na CALD.
(6) Kymriah
Ụlọ ọrụ: Novartis mepụtara.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na August 2017.
Ngosipụta: Ọgwụgwọ nke precursor B-cell nnukwu lymphoblastic leukemia (ALL) na nlọghachite na refractory DLBCL.
Nkọwa: Kymriah bụ ọgwụ lentiviral in vitro gene therapy, ọgwụgwọ CAR-T mbụ akwadoro maka ịre ahịa n'ụwa, na-ezubere CD19, yana iji 4-1BB co-stimulatory factor.A na-ere ya na $475,000 na US na $313,000 na Japan.
(7)Yescarta
Ụlọ ọrụ: Kite Pharma, onye enyemaka Gilead (GILD) mebere ya.
Oge ịzụ ahịa: FDA kwadoro maka ịzụ ahịa na October 2017;Fosun Kite webatara teknụzụ Yescarta site na Kite Pharma wee mepụta ya na China mgbe ọ nwetasịrị ikike.Ekwenyere maka ndebanye aha na obodo.
Ngosipụta: Maka ọgwụgwọ nke nlọghachi azụ ma ọ bụ refractory nnukwu B-cell lymphoma.
Okwu: Yescarta bụ ọgwụgwọ retroviral in vitro gene therapy, nke bụ ọgwụgwọ CAR-T nke abụọ akwadoro n'ụwa.Ọ na-elekwasị anya CD19 ma nakweere costimulator nke CD28.A na-ere ya na $373,000 na United States.
(8) Tecartus
Ụlọ ọrụ: Gilead (GILD) mebere ya.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na Julaị 2020.
Ngosipụta: Maka nlọghachite ma ọ bụ refractory mantle cell lymphoma.
Okwu: Tecartus bụ usoro ọgwụgwọ cell CAR-T nke na-achọ CD19, na ọ bụ ọgwụgwọ CAR-T nke atọ akwadoro maka ire ahịa n'ụwa.
(9) Breyanzi
Ụlọ ọrụ: Bristol-Myers Squibb (BMS) mebere ya.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na February 2021.
Ngosipụta: Nlaghachi ma ọ bụ refractory (R/R) nnukwu lymphoma B-cell (LBCL).
Nkwupụta: Breyanzi bụ ọgwụgwọ mkpụrụ ndụ in vitro dabere na lentivirus, ọgwụgwọ CAR-T nke anọ akwadoro maka ịre ahịa n'ụwa, na-ezubere CD19.Nkwenye nke Breyanzi bụ ihe dị ịrịba ama maka Bristol-Myers Squibb na ngalaba nke immunotherapy cellular, nke ọ nwetara mgbe ọ nwetara Celgene maka ijeri $ 74 na 2019.
(10) Abecma
Ụlọ ọrụ: Ụlọ ọrụ-mepụtara site na Bristol-Myers Squibb (BMS) na bluebird bio.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na Machị 2021.
Ngosipụta: nlọghachi azụ ma ọ bụ refractory multiple myeloma.
Okwu: Abecma bụ ọgwụgwọ mkpụrụ ndụ in vitro dabere na lentivirus, usoro ọgwụgwọ mkpụrụ ndụ CAR-T mbụ nke ụwa na-elekwasị anya BCMA, yana ọgwụgwọ CAR-T nke ise nke FDA kwadoro.Ụkpụrụ nke ọgwụ ahụ bụ igosipụta chimeric BCMA nnabata na mkpụrụ ndụ T nke onye ọrịa site na mgbanwe mkpụrụ ndụ ihe nketa lentivirus na vitro.Ọgwụgwọ iji kpochapụ mkpụrụ ndụ T na-abụghị mkpụrụ ndụ ihe nketa na ndị ọrịa, wee nwetaghachi mkpụrụ ndụ T gbanwere, nke na-achọ ma na-egbu BCMA na-egosipụta mkpụrụ ndụ kansa na ndị ọrịa.
(11) Libmeldy
Ụlọ ọrụ: Orchard Therapeutics mepụtara.
Oge ịre ahịa: European Union kwadoro maka ndepụta na Disemba 2020.
Ngosipụta: Maka ọgwụgwọ metachromatic leukodystrophy (MLD).
Okwu: Libmeldy bụ usoro ọgwụgwọ mkpụrụ ndụ ihe nketa dabere na sel CD34+ na-akpaghasị mkpụrụ ndụ ihe nketa na vitro site na lentivirus.Data ụlọọgwụ na-egosi na otu infusion intravenous nke Libmeldy nwere ike gbanwee usoro mmalite mmalite nke MLD nke ọma ma e jiri ya tụnyere moto siri ike na nhụta ọgụgụ isi na ndị ọrịa a na-agwọghị nke otu afọ.
(12) Benoda
Ụlọ ọrụ: WuXi Giant Nuo mebere ya.
Oge ịre ahịa: NMPA kwadoro na Septemba 2021.
Ngosipụta: Ọgwụgwọ nke ndị ọrịa toro eto nwere nlọghachi azụ ma ọ bụ refractory nnukwu B-cell lymphoma (r / r LBCL) mgbe usoro nke abụọ ma ọ bụ karịa usoro ọgwụgwọ.
Nkwupụta: Beinoda bụ ọgwụgwọ mkpụrụ ndụ ihe mgbochi CD19 CAR-T, ọ bụkwa isi ngwaahịa nke ụlọ ọrụ WuXi Juro.Ọ bụ ngwaahịa nke abụọ a kwadoro na China, belụsọ maka ngbanwe / ịgbanyụ na-agbagha, manthocytic leukemia (WLBPHY) ymphoblastic leukemia (niile).
(13) CARVYKTI
Ụlọ ọrụ: Ngwaahịa mbụ Legend Biotech kwadoro maka ịzụ ahịa.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na February 2022.
Ngosipụta: maka ọgwụgwọ nlọghachi azụ ma ọ bụ refractory multiple myeloma (R / R MM).
Nkwupụta: CARVYKTI (ciltacabtagene autoleucel, Cilta-cel dị mkpụmkpụ) bụ ọgwụgwọ mkpụrụ ndụ mgbochi nke CAR-T nwere ọgwụ mgbochi abụọ nwere otu ngalaba na-elekwasị anya na antigen B-cell maturation antigen (BCMA).Data na-egosi na CARVYKTI N'ime ndị ọrịa nwere nlọghachi azụ ma ọ bụ refractory multiple myeloma bụ ndị natara ọgwụgwọ anọ ma ọ bụ karịa tupu ọgwụgwọ (gụnyere proteasome inhibitors, immunomodulators na anti-CD38 monoclonal antibodies), n'ozuzu nzaghachi nke 98% ka egosiri.
(14)Ebvallo
Ụlọ ọrụ: Atara Biotherapeutics mepụtara.
European Commission (EC) maka ịre ahịa na Disemba 2022, ọ bụ usoro ọgwụgwọ T cell mbụ nke ụwa kwadoro maka ịzụ ahịa.
Ngosipụta: Dị ka monotherapy maka nje Epstein-Barr (EBV) metụtara ọrịa lymphoproliferative post-transplantation (EBV+PTLD), ndị ọrịa na-anata ọgwụgwọ ga-abụrịrị ndị okenye na ụmụaka karịrị afọ 2 bụ ndị natara mbụ ma ọ dịkarịa ala otu ọgwụgwọ ọgwụ ọzọ.
Nkwupụta: Ebvallo bụ usoro ọgwụgwọ mkpụrụ ndụ T-cell zuru ụwa ọnụ nke EBV nke na-elekwasị anya ma wepụ mkpụrụ ndụ nje EBV n'ụzọ amachibidoro HLA.Nkwenye nke ọgwụgwọ a dabere na nsonaazụ nke ọmụmụ ihe ọmụmụ ụlọ ọgwụ dị mkpa nke 3, yana nsonaazụ gosiri na ORR nke otu HCT na otu SOT bụ 50%.Ọnụego mgbaghara zuru oke (CR) bụ 26.3%, ọnụego mgbaghara elere anya (PR) bụ 23.7%, na oge mgbaghara (TTR) bụ ọnwa 1.1.N'ime ndị ọrịa 19 nwetara mgbaghara, 11 nwere oge nzaghachi (DOR) karịa ọnwa 6.Na mgbakwunye, n'ihe gbasara nchekwa, ọ nweghị mmeghachi omume ọjọọ dịka ọrịa graft-versus-host (GvHD) ma ọ bụ ọrịa ntọhapụ cytokine metụtara Ebvallo mere.
2. In vivo gene therapy dabere na viral vectors
(1) Gendicine/Jin Sheng
Ụlọ ọrụ: Shenzhen Saibainuo Company mepụtara.
Oge ịre ahịa: Ekwenyere ka edepụta ya na China na 2003.
Ngosipụta: Maka ọgwụgwọ nke isi na olu squamous cell carcinoma.
Mara: Recombinant human p53 adenovirus injection Gendicine/Jinyousheng bụ ọgwụ adenovirus vector gene therapy nwere ikike ikike ọgụgụ isi nke onwe nke ụlọ ọrụ Shenzhen Saibainuo nwere.Ụdị mmadụ 5 adenovirus mejupụtara ụdị adenovirus mmadụ nke 5. Nke mbụ bụ usoro bụ isi maka mmetụta mgbochi tumor nke ọgwụ, na nke ikpeazụ na-arụ ọrụ dị ka onye na-ebu.The adenovirus vector na-eburu ọgwụgwọ ọgwụgwọ p53 n'ime cell ezubere iche, na-egosipụta tumor suppressor gene p53 na cell ezubere iche, na mkpụrụ ndụ ihe nketa ya Ngwaahịa ahụ nwere ike ịkwalite ụdị ọrịa cancer dị iche iche ma na-achịkwa ọrụ nke ụdị oncogenes dị iche iche, si otú ahụ na-eme ka ọkpụkpụ anụ ahụ na-ebelata ihe kpatara ya na-egbu egbu.
(2) Rigvir
Ụlọ ọrụ: Ụlọ ọrụ Latima mepụtara, Latvia.
Oge ndepụta: akwadoro maka ndepụta na Latvia na 2004.
Ngosipụta: Maka ọgwụgwọ nke melanoma.
Okwu: Rigvir bụ ọgwụgwọ mkpụrụ ndụ ihe nketa dabere na mkpụrụ ndụ ihe nketa ECHO-7 enterovirus vector gbanwere.Ka ọ dị ugbu a, a nakweere ọgwụ ahụ na Latvia, Estonia, Poland, Armenia, Belarus, wdg, ma na-edebanye aha EMA na mba EU.Ọnọdụ ụlọ ọgwụ n'ime afọ iri gara aga egosila na nje Rigvir oncolytic dị mma ma dị irè, ma nwee ike ịbawanye ọnụọgụ ndụ nke ndị ọrịa melanoma ugboro 4-6.Tụkwasị na nke a, ọgwụgwọ a na-emetụtakwa ụdị ọrịa cancer ndị ọzọ dị iche iche, gụnyere ọrịa cancer colorectal, cancer pancreatic, cancer cancer, cancer cancer, cancer cancer, prostate cancer, cancer cancer, uterine cancer, lymphosarcoma, etc.
(3) Oncorine
Ụlọ ọrụ: Ụlọ ọrụ Shanghai Sanwei Biological Mepụtara.
Oge ịre ahịa: Ekwenyere ka edepụta ya na China na 2005.
Ngosipụta: ọgwụgwọ etuto isi na olu, ọrịa imeju, ọrịa pancreatic, kansa cervical na ọrịa cancer ndị ọzọ.
Okwu: Oncorine (安科瑞) bụ ngwaahịa ọgwụgwọ mkpụrụ ndụ oncolytic na-eji adenovirus dị ka onye na-ebu.A na-enweta adenovirus oncolytic, nke nwere ike ịmegharị kpọmkwem na p53 gene erughi ma ọ bụ ụbụrụ na-adịghị mma, na-eduga na lysis nke mkpụrụ ndụ tumo, si otú ahụ na-egbu mkpụrụ ndụ tumo.na-emebighị sel nkịtị.Ọmụmụ ụlọ ọgwụ egosila na Ankerui nwere nchekwa dị mma na ịdị irè maka ụdị ụbụrụ ọjọọ dị iche iche.
(4) Glybera
Ụlọ ọrụ: UniQre mebere ya.
Oge ịre ahịa: akwadoro maka ndepụta na Europe na 2012.
Ngosipụta: Ọgwụgwọ ụkọ lipoprotein lipase (LPLD) nwere nnukwu ọrịa pancreatitis ma ọ bụ ugboro ugboro n'agbanyeghị nri abụba amachibidoro.
Nkwupụta: Glybera (alipogene tiparvovec) bụ ọgwụ ọgwụgwọ mkpụrụ ndụ ihe nketa dabere na AAV, nke na-eji AAV dị ka onye na-ebu ibu na-ebufe mkpụrụ ndụ ọgwụgwọ LPL n'ime mkpụrụ ndụ akwara, nke mere na mkpụrụ ndụ kwekọrọ na ya nwere ike mepụta ụfọdụ lipoprotein lipase, Iji belata ọrịa ahụ, ọgwụgwọ a na-arụ ọrụ ogologo oge nwere ike ịdịgide ruo ọtụtụ afọ.Ewepụrụ ọgwụ ahụ n'ahịa na 2017. Ihe kpatara nkwụsị ya nwere ike jikọta ya na ihe abụọ: ọnụ ahịa dị elu na oke ahịa ahịa.Ọnụ ego ọgwụgwọ ọgwụ a na-eri dị elu ruru nde US $ 1, ma ọ bụ naanị otu onye ọrịa zụrụ ma jiri ya mee ihe ruo ugbu a.Ọ bụ ezie na ụlọ ọrụ ịnshọransị ahụike akwụghachila US$900,000 maka ya, ọ bụkwa ibu dịtụ ibu maka ụlọ ọrụ ịnshọransị ahụ.Tụkwasị na nke ahụ, ihe ngosi ndị ezubere iche maka ọgwụ ahụ dị oke ụkọ, yana ọnụ ọgụgụ ihe dị ka 1 n'ime nde 1 na ọnụ ọgụgụ dị elu nke nchọpụta na-ezighị ezi.
(5) Ikwu okwu
Ụlọ ọrụ: Amgen mepụtara.
Oge ịre ahịa: Na 2015, akwadoro ka edepụta ya na United States na European Union.
Ngosipụta: Ọgwụgwọ nke ọnya melanoma nke enweghị ike iwepụ kpamkpam site na ịwa ahụ.
Okwu: Imlygic bụ nje herpes simplex attenuated ụdị 1 nke ejirila teknụzụ mkpụrụ ndụ gbanwee (ihichapụ mkpụrụ ndụ ICP34.5 na ICP47 ya, yana itinye mmadụ granulocyte macrophage colony-stimulating factor GM-CSF gene n'ime nje) (HSV-1) nje oncolytic bụ nje mbụ nke FDA-approv.Usoro nchịkwa bụ injection intralesional, nke nwere ike ịgbanye ozugbo na ọnya melanoma iji mee ka ọkpụkpụ nke mkpụrụ ndụ tumor, hapụ antigens na-enweta tumor na GM-CSF, ma kwalite nzaghachi mgbochi tumor.
(6) Luxturna
Ụlọ ọrụ: Spark Therapeutics, onye enyemaka nke Roche mepụtara.
Oge ịzụ ahịa: FDA kwadoro ya maka ire ere na 2017, wee kwado ya maka ịzụ ahịa na Europe na 2018.
Ngosipụta: Maka ọgwụgwọ ụmụaka na ndị okenye bụ ndị furu efu n'ihi mmụgharị mkpụrụ ndụ RPE65 okpukpu abụọ mana jigide ọnụ ọgụgụ zuru oke nke mkpụrụ ndụ retinal nwere ike ime.
Okwu: Luxturna bụ usoro ọgwụgwọ mkpụrụ ndụ ihe nketa dabere na AAV nke a na-enye site na ntụtụ subretinal.Usoro ọgwụgwọ mkpụrụ ndụ ihe nketa na-eji AAV2 dị ka onye na-ebu ibu iji webata ọrụ nke mkpụrụ ndụ RPE65 nkịtị n'ime sel retinal nke onye ọrịa, nke mere na mkpụrụ ndụ kwekọrọ na-egosipụta protein RPE65 nkịtị, na-eme ka enweghi protein RPE65 nke onye ọrịa ahụ, si otú ahụ mee ka ọhụụ onye ọrịa dịkwuo mma.
(7) Zolgensma
Ụlọ ọrụ: AveXis mepụtara, enyemaka nke Novartis.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na Mee 2019.
Ngosipụta: Ọgwụgwọ nke Atrophy Muscular Atrophy (SMA) ndị ọrịa nọ n'okpuru afọ 2.
Nkọwa: Zolgensma bụ ọgwụgwọ mkpụrụ ndụ ihe nketa dabere na vector AAV.Ọgwụ a bụ naanị atụmatụ ọgwụgwọ otu oge maka atrophy muscular spinal nke akwadoro maka ire ahịa n'ụwa.Mwepụta nke ọgwụ ahụ na-emepe oge ọhụrụ na ọgwụgwọ nke atrophy muscular spinal.ibe, bụ ọganihu dị ịrịba ama.Usoro ọgwụgwọ mkpụrụ ndụ ihe nketa a na-eji vector scAAV9 ewebata mkpụrụ ndụ SMN1 nkịtị n'ime onye ọrịa site na infusion intravenous iji mepụta protein SMN1 nkịtị, si otú a na-emeziwanye ọrụ nke mkpụrụ ndụ emetụtara dị ka neurons moto.N'ụzọ dị iche, ọgwụ SMA Spinraza na Evrysdi chọrọ usoro ọgwụgwọ ugboro ugboro ogologo oge.A na-enye Spinraza site n'ịgba ọgwụ ọkpụkpụ azụ kwa ọnwa anọ, Evrysdi bụ ọgwụ ọnụ kwa ụbọchị.
(8) Na-akpachapụ anya
Ụlọ ọrụ: Mepụtara ya Daiichi Sankyo Company Limited (TYO: 4568).
Oge ịre ahịa: Nkwenye ọnọdụ sitere na Ministry of Health, Labour and Welfare (MHLW) na June 2021.
Ngosipụta: Maka ọgwụgwọ glioma dị njọ.
Nkwupụta: Delytact bụ ngwaahịa ọgwụgwọ mkpụrụ ndụ nje oncolytic nke anọ akwadoro n'ụwa niile, yana ngwaahịa nje oncolytic izizi akwadoro maka ọgwụgwọ glioma na-adịghị mma.Delytact bụ nje virus oncolytic nke herpes simplex ụdị 1 (HSV-1) nke Dr. Todo na ndị ọrụ ibe mepụtara.Delytact na-ewebata mmụgharị nhichapụ ndị ọzọ n'ime genome G207 nke ọgbọ nke abụọ HSV-1, na-eme ka mmegharị ya dị na mkpụrụ ndụ kansa na ntinye nke nzaghachi mgbochi tumor ka ọ na-edobe nchekwa dị elu.Delytact bụ ọgbọ mbụ oncolytic HSV-1 nke na-eme nyocha ụlọ ọgwụ ugbu a.Nkwenye nke Delytact na Japan dabere na nnwale ụlọ ọgwụ nwere otu ogwe aka.N'ime ndị ọrịa nwere glioblastoma ugboro ugboro, Delytact nwetara isi njedebe nke ndụ ndụ otu afọ, nsonaazụ ya gosipụtara na Delytact gosipụtara arụmọrụ ka mma ma e jiri ya tụnyere G207.Ike mmeghari siri ike na ọrụ antitumor dị elu.Nke a dị irè n'ụdị tumor siri ike nke ara, prostate, schwannomas, nasopharyngeal, hepatocellular, colorectal, malignant peripheral nerve sheath tumors, na thyroid cancer.
(9) Ugboro
Ụlọ ọrụ: Mepụtara site na PTC Therapeutics, Inc. (NASDAQ: PTCT).
Oge ịre ahịa: European Union kwadoro maka ịre ahịa na Julaị 2022.
Ngosipụta: Maka ụkọ L-amino acid decarboxylase (AADC), a kwadoro ya maka ọgwụgwọ ndị ọrịa gbara ọnwa 18 na karịa.
Okwu: Upstaza™ (eladocagene exuparvovec) bụ ọgwụgwọ mkpụrụ ndụ ihe nketa in vivo nwere ụdị nje metụtara adeno 2 (AAV2) dị ka onye na-ebu ya.Ndị ọrịa na-arịa ọrịa n'ihi mmụgharị na mkpụrụ ndụ ihe nketa na-etinye enzyme AADC.AAV2 na-ebu mkpụrụ ndụ dị mma na-edobe enzyme AADC.Ụdị nkwụghachi ụgwọ mkpụrụ ndụ ihe nketa na-enweta mmetụta ọgwụgwọ.Na tiori, otu nchịkwa dị irè ruo ogologo oge.Ọ bụ usoro ọgwụgwọ mkpụrụ ndụ ihe nketa nke mbụ a na-agbanye ozugbo n'ime ụbụrụ.Ikike ịre ahịa na-emetụta mba 27 niile EU, yana Iceland, Norway na Liechtenstein.
(10) Roctavian
Ụlọ ọrụ: BioMarin Pharmaceutical (BioMarin) mepụtara.
Oge ịre ahịa: Ndị European Union kwadoro maka ịre ahịa n'August 2022;ikike ịre ahịa nke UK Medicines and Healthcare Product Administration (MHRA) na Nọvemba 2022.
Ngosipụta: Maka ọgwụgwọ nke ndị ọrịa toro eto nwere nnukwu hemophilia A na-enweghị akụkọ ihe mere eme nke mgbochi FVIII ma na-adịghị mma maka ọgwụ mgbochi AAV5.
Nkwuputa: Roctavian (valoctocogene roxaparvovec) na-eji AAV5 dị ka vector ma na-eji onye nkwalite imeju mmadụ kpọmkwem HLP iji mee ka nkwupụta nke coagulation factor mmadụ VIII (FVIII) na ngalaba B ehichapụ.Mkpebi European Commission kwadoro ịre ahịa nke valoctocogene roxaparvovec dabere na mkpokọta data nke ọrụ mmepe ụlọ ọgwụ ọgwụ.N'ime ha, nsonaazụ nke usoro nke atọ nke ụlọ ọgwụ GENER8-1 gosiri na e jiri ya tụnyere data nke afọ tupu edebanye aha ya, mgbe otu infusion nke valoctocogene roxaparvovec gasịrị, ọnụ ọgụgụ ọbara ọgbụgba kwa afọ nke isiokwu (ABR) na-ebelata nke ukwuu, ugboro ole iji recombinant coagulation factor VIII (F8) nkwadebe protein na-ebelata, ma ọ bụ na-abawanye ọrụ nke F8.Mgbe izu anọ nke ọgwụgwọ gasịrị, ọnụ ọgụgụ ojiji F8 nke isiokwu ahụ kwa afọ yana ABR chọrọ ọgwụgwọ belatara site na 99% na 84%, n'otu n'otu, ọdịiche ahụ dị ịrịba ama na ọnụ ọgụgụ (p<0.001).Profaịlụ nchekwa ahụ dị mma, ọ nweghịkwa isiokwu nwetara ihe mgbochi F8, mmerụ ahụ ma ọ bụ mmetụta thrombosis, na enweghị mmemme ọjọọ metụtara ọgwụgwọ (SAEs).
(11) Hemgenix
Ụlọ ọrụ: UniQure Corporation mepụtara.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na Nọvemba 2022.
Ngosipụta: Maka ọgwụgwọ ndị okenye nwere hemophilia B.
Okwu: Hemgenix bụ ọgwụgwọ mkpụrụ ndụ ihe nketa dabere na vector AAV5.Emebere ọgwụ a na coagulation factor IX (FIX) gene variant FIX-Padua, nke a na-enye n'ime intravenously.Mgbe nchịkwa gasịrị, mkpụrụ ndụ ihe nketa nwere ike igosipụta FIX coagulation factor na imeju na nzuzo Mgbe ịbanye n'ọbara iji rụọ ọrụ coagulation, iji nweta nzube nke ọgwụgwọ, usoro iwu, otu nchịkwa dị irè ruo ogologo oge.
(12) Adstiladrin
Ụlọ ọrụ: Ferring Pharmaceuticals mepụtara.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na Disemba 2022.
Ngosipụta: Maka ọgwụgwọ nke nnukwu ihe ize ndụ nke na-abụghị nke anụ ahụ na-adịghị emerụ ahụ (NMIBC) adịghị anabata Bacillus Calmette-Guerin (BCG).
Nkwupụta: Adstiladrin bụ usoro ọgwụgwọ mkpụrụ ndụ ihe nketa na-adabere na vector adenoviral na-abụghị nke na-emegharị ahụ, nke nwere ike overexpress interferon alfa-2b protein n'ime mkpụrụ ndụ ebumnuche, ma na-enye ya site na catheter urinary n'ime eriri afo (a na-enye ya otu ugboro kwa ọnwa atọ), vector nje nwere ike ibunye nke ọma n'ime sel nke mgbidi eriri afo, wee mebie protein nke interferon.Usoro ọgwụgwọ mkpụrụ ndụ ihe nketa a na-eme ka mkpụrụ ndụ mgbidi nke eriri afo nke onye ọrịa ghọọ obere “ụlọ ọrụ” nke na-emepụta interferon, si otú ahụ na-eme ka onye ọrịa nwee ike ịlụso ọrịa cancer ọgụ.
A tụlere nchekwa na ịdị irè nke Adstiladrin na nyocha ụlọ ọgwụ dị iche iche gụnyere ndị ọrịa 157 nwere nnukwu ihe ize ndụ BCG-anaghị anabata NMIBC.Ndị ọrịa na-anata Adstiladrin kwa ọnwa atọ ruo ọnwa iri na abụọ, ma ọ bụ ruo mgbe nsi na-anabataghị ọgwụgwọ ma ọ bụ nlọghachi nke ọkwa NMIBC dị elu.N'ozuzu, pasent 51 nke ndị ọrịa debanyere aha na Adstiladrin nwetara nzaghachi zuru oke (mpụta nke ihe ịrịba ama niile nke ọrịa cancer na-ahụ na cystoscopy, anụ ahụ biopsy, na mmamịrị).
3. Obere ọgwụ nucleic acid
(1) Vitravene
Ụlọ ọrụ: Ejikọtara ọnụ nke Ionis Pharma (nke bụbu Isis Pharma) na Novartis.
Oge ịzụ ahịa: Na 1998 na 1999, FDA na EU EMA kwadoro ya maka ịzụ ahịa.
Ngosipụta: Maka ọgwụgwọ cytomegalovirus retinitis na ndị ọrịa nwere nje HIV.
Nkwuputa: Vitravene bụ ọgwụ oligonucleotide antisense, nke bụ ọgwụ oligonucleotide mbụ kwadoro maka ịzụ ahịa n'ụwa.Na ọkwa mbụ nke ndepụta, ahịa ahịa maka ọgwụ mgbochi CMV dị ngwa ngwa;emesia, n'ihi mmepe nke ọgwụgwọ antiretroviral na-arụsi ọrụ ike, ọnụ ọgụgụ nke CMV gbadara nke ọma.N'ihi agụụ ahịa dị nwayọ, ewepụtara ọgwụ ahụ na 2002 na 2006 iwepụ na mba EU na United States.
(2) Macugen
Ụlọ ọrụ: Pfizer na Eyetech mebere ya.
Oge ịre ahịa: Ekwenyere maka ịdebanye aha na United States na 2004.
Ngosipụta: Maka ọgwụgwọ nke ọrịa macular degeneration metụtara afọ neovascular.
Okwu: Macugen bụ ọgwụ oligonucleotide nke pegylated emezigharịrị, nke nwere ike ịche ma jikọta vaskụla endothelial growth factor (VEGF165 subtype), na usoro nchịkwa bụ ịgba ọgwụ intravitreal.
(3) Defitelio
Ụlọ ọrụ: Jazz Pharmaceuticals mepụtara.
Oge ịre ahịa: European Union kwadoro ya maka ịzụ ahịa na 2013 ma kwadoro ya na FDA maka ịzụ ahịa na March 2016.
Ngosipụta: Maka ọgwụgwọ ọrịa ịba ọcha n'anya veno-occlusive nke metụtara renal ma ọ bụ pulmonary dysfunction mgbe transplantation hematopoietic stem cell.
Okwu: Defitelio bụ ọgwụ oligonucleotide, nke bụ ngwakọta nke oligonucleotides nwere ihe plasmin.Ewepụrụ n'ahịa na 2009 maka ebumnuche azụmahịa.
(4) Kynamro
Ụlọ ọrụ: Ionis Pharma na Kastle mebere ya.
Oge ịzụ ahịa: Na 2013, a kwadoro ya maka ịzụ ahịa na United States dị ka ọgwụ na-enweghị mgbei.
Ngosipụta: Maka ọgwụgwọ adjuvant nke homozygous familial hypercholesterolemia.
Nkwupụta: Kynamro bụ ọgwụ oligonucleotide antisense, nke bụ antisense oligonucleotide na-ezubere apo B-100 mRNA mmadụ.A na-enye Kynamro dị ka 200 mg subcutaneously otu ugboro n'izu.
(5) Spinraza
Ụlọ ọrụ: Ionis Pharmaceuticals mepụtara.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na Disemba 2016.
Ngosipụta: Maka ọgwụgwọ nke spinal muscular atrophy (SMA).
Okwu: Spinraza (nusinersen) bụ ọgwụ antisense oligonucleotide.Site na ijikọ na saịtị mgbawa nke SMN2 exon 7, Spinraza nwere ike gbanwee RNA cleavage nke SMN2 gene, si otú ahụ na-abawanye mmepụta nke protein SMN na-arụ ọrụ nke ọma.N'August 2016, BIOGEN gosipụtara nhọrọ ya iji nweta ikike zuru ụwa ọnụ na Spinraza.Spinraza naanị malitere nnwale ụlọ ọgwụ mbụ ya na ụmụ mmadụ na 2011. N'ime naanị afọ 5, FDA kwadoro ya maka ịre ahịa na 2016, nke na-egosipụta nnabata zuru oke nke FDA maka ịdị irè ya.A kwadoro ọgwụ ahụ maka ịzụ ahịa na China na Eprel 2019. Usoro nkwenye niile maka Spinraza na China bụ ihe na-erughị ọnwa 6, ọ bụkwa afọ 2 na ọnwa 2 kemgbe a kwadoro Spinraza na United States.Ọsọ nke ndepụta na China adịlarị ngwa ngwa.Nke a bụkwa n'ihi n'eziokwu na Center for Drug Evaluation nyere "Nrịbama na-ebipụta ndepụta nke mbụ ogbe nke esenidụt ọhụrụ ọgwụ ngwa ngwa mkpa na Clinical Practice" na November 1, 2018, na e tinyere na mbụ ogbe nke 40 mba ọzọ ọgwụ ọhụrụ maka ngwa ngwa nyochaa, n'ime nke Spinraza họọrọ.
(6) Ọpụpụ 51
Ụlọ ọrụ: AVI BioPharma mepụtara (nke emechara aha ya bụ Sarepta Therapeutics).
Oge ịzụ ahịa: Na Septemba 2016, FDA kwadoro ya maka ịzụ ahịa.
Ngosipụta: Maka ọgwụgwọ nke Duchenne muscular dystrophy (DMD) na exon 51 skipping gene mutation na DMD gene.
Nkwupụta: Exondys 51 bụ ọgwụ antisense oligonucleotide, antisense oligonucleotide nwere ike jikọta ya na ọnọdụ exon 51 nke pre-mRNA nke DMD gene, na-akpata nguzobe nke mRNA tozuru oke, akụkụ nke exon 51 na-egbochi Excision, si otú ahụ na-edozi akụkụ nke mRNA ọgụgụ isi nke na-enyere aka imeziwanye usoro ọgụgụ nke mRNA, na-enyere aka imeziwanye usoro ọgụgụ nke mRNA, na-enyere aka imeziwanye usoro ọgụgụ nke mRNA na-arụ ọrụ nke ọma. mgbaàmà nke onye ọrịa.
(7) Tegsedi
Ụlọ ọrụ: Ionis Pharmaceuticals mepụtara.
Oge ịre ahịa: European Union kwadoro ya maka ire ahịa na July 2018.
Ngosipụta: Maka ọgwụgwọ transthyretin amyloidosis (hATTR) nke ketara eketa.
Nkwupụta: Tegsedi bụ ọgwụ antisense oligonucleotide na-ezubere transthyretin mRNA.Ọ bụ ọgwụ izizi akwadoro n'ụwa maka ọgwụgwọ haTTR.A na-enye ya site na ntụtụ subcutaneous.Ọgwụ ahụ na-ebelata mmepụta nke protein ATTR site n'ịchụso mRNA nke transthyretin (ATTR), ma nwee ezigbo uru-ihe ize ndụ na ọgwụgwọ ATTR, na neuropathy nke onye ọrịa na ndụ ndụ emeela ka ọ dịkwuo mma, ọ dabara na ụdị mgbanwe TTR, Ọ bụghị ọkwa ọrịa ma ọ bụ ọnụnọ nke cardiomyopathy dị mkpa.
(8) Onpattro
Ụlọ ọrụ: Ụlọ ọrụ Alnylam na Sanofi Corporation jikọtara ọnụ.
Oge ịre ahịa: Ekwenyere maka ndepụta na United States na 2018.
Ngosipụta: Maka ọgwụgwọ transthyretin amyloidosis (hATTR) nke ketara eketa.
Okwu: Onpattro bụ ọgwụ siRNA na-ezubere transthyretin mRNA, nke na-ebelata mmepụta nke protein ATTR na imeju ma na-ebelata mkpokọta amyloid n'ime irighiri akwara site na ikwado mRNA nke transthyretin (ATTR), si otú ahụ na-emeziwanye ma na-ebelata mgbaàmà ọrịa.
(9) Givlaari
Ụlọ ọrụ: Alnylam Corporation mepụtara.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na Nọvemba 2019.
Ngosipụta: Maka ọgwụgwọ nnukwu hepatic porphyria (AHP) na ndị okenye.
Nkwupụta: Givlaari bụ ọgwụ siRNA, nke bụ ọgwụ siRNA nke abụọ akwadoro maka ire ahịa mgbe Onpattro gachara.Ụzọ nchịkwa bụ ntụtụ subcutaneous.Ọgwụ ahụ lekwasịrị anya mRNA nke protein ALAS1, yana ọgwụgwọ kwa ọnwa na Givlaari nwere ike belata ọkwa ALAS1 na imeju na-adịgide adịgide, si otú a na-ebelata ọkwa neurotoxic ALA na PBG n'ụdị nkịtị, si otú ahụ belata mgbaàmà nke ọrịa onye ọrịa.Ihe omuma a gosiputara na ndi oria Givlaari mebere nwere mbelata 74% n’onu ogugu ọdịdọ ma e jiri ya tụnyere otu placebo.
(10) Vyondys53
Ụlọ ọrụ: Sarepta Therapeutics mepụtara.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na Disemba 2019.
Ngosipụta: Maka ọgwụgwọ nke ndị ọrịa DMD nwere dystrophin gene exon 53 splicing mutation.
Nkwuputa: Vyondys 53 bụ ọgwụ oligonucleotide antisense, nke na-elekwasị anya n'usoro nhazi nke dystrophin pre-mRNA.Exon 53 ka agbajichara akụkụ ya, ya bụ, ọ nọghị na mRNA tozuru oke, ma emebere ya ka ọ mepụta dystrophin gbajiri agbaji mana ọ ka na-arụ ọrụ, wee si otú a kwalite ikike mmega ahụ na ndị ọrịa.
(11) Waylivra
Ụlọ ọrụ: Mepụtara site na Ionis Pharmaceuticals na enyemaka ya Akcea Therapeutics.
Oge ịre ahịa: Ndị na-ahụ maka ọgwụ na Europe (EMA) kwadoro ya maka ire ahịa na Mee 2019.
Ngosipụta: Dị ka ọgwụgwọ adjuvant na mgbakwunye na njikwa nri na ndị okenye nwere ọrịa familial chylomicronemia syndrome (FCS).
Nkwupụta: Waylivra bụ ọgwụ oligonucleotide antisense, nke bụ ọgwụ izizi akwadoro maka ire ahịa n'ụwa maka ọgwụgwọ FCS.
(12) Leqvio
Ụlọ ọrụ: Novartis mepụtara.
Oge ịre ahịa: European Union kwadoro maka ịre ahịa na Disemba 2020.
Ngosipụta: Maka ọgwụgwọ nke ndị okenye nwere hypercholesterolemia bụ isi (heterozygous familial and non-familial) ma ọ bụ agwakọta dyslipidemia.
Okwu: Leqvio bụ ọgwụ siRNA na-ezubere PCSK9 mRNA.Ọ bụ ọgwụgwọ siRNA mbụ n'ụwa maka ibelata cholesterol (LDL-C).A na-enye ya site na ntụtụ subcutaneous.Ọgwụ ahụ na-ebelata ọkwa nke protein PCSK9 site na ndabichi RNA, si otú ahụ belata ọkwa LDL-C.Data ụlọ ọgwụ na-egosi na maka ndị ọrịa na-enweghị ike ibelata ọkwa LDL-C ruo ọkwa ebumnuche mgbe ọgwụgwọ ya na statins kacha anabatara, Leqvio nwere ike belata LDL-C ihe dịka 50%.
(13)Oxlumo
Ụlọ ọrụ: Alnylam Pharmaceuticals mepụtara.
Oge ịre ahịa: European Union kwadoro maka ịre ahịa na Nọvemba 2020.
Ngosipụta: Maka ọgwụgwọ nke isi hyperoxaluria ụdị 1 (PH1).
Okwu: Oxlumo bụ ọgwụ siRNA na-ezubere hydroxyacid oxidase 1 (HAO1) mRNA, na usoro nchịkwa bụ ntụtụ subcutaneous.Emepụtara ọgwụ ahụ site na iji kemịkalụ nkwụsi ike kachasị ọhụrụ nke Alnylam, teknụzụ njikọ njikọ ESC-GalNAc, nke na-enyere siRNA a na-ahụ maka subcutaneously nwee nnọgidesi ike na ike dị ukwuu.Ọgwụ ahụ na-eweda ma ọ bụ na-egbochi hydroxyacid oxidase 1 (HAO1) mRNA, na-ebelata ọkwa nke glycolate oxidase na imeju, wee na-eri ihe ndị a chọrọ maka mmepụta nke oxalate, na-ebelata mmepụta oxalate iji chịkwaa ọganihu nke ọrịa ahụ na ndị ọrịa ma melite mgbaàmà ọrịa.
(14) Viltepso
Ụlọ ọrụ: NS Pharma mebere ya, onye enyemaka nke Nippon Shinyaku.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa n'August 2020.
Ngosipụta: Maka ọgwụgwọ Duchenne muscular dystrophy (DMD) na exon 53 skipping gene mutation na DMD gene.
Nkwupụta: Viltepso bụ ọgwụ antisense oligonucleotide nke nwere ike jikọta ya na ọnọdụ exon 53 nke pre-mRNA nke mkpụrụ ndụ DMD, na-eme ka akụkụ nke exon 53 wepụ ya mgbe e guzobechara mRNA tozuru okè, si otú ahụ na-agbazi akụkụ nke mRNA ịgụ akwụkwọ igbe ahụ na-enyere ndị ọrịa aka ịmepụta ụfọdụ ụdị arụ ọrụ nke protein dystrophin na-eme ka protein dị mkpụmkpụ karịa nke ahụ.
(15) Afọ 45
Ụlọ ọrụ: Sarepta Therapeutics mepụtara.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na February 2021.
Ngosipụta: Maka ọgwụgwọ Duchenne muscular dystrophy (DMD) na exon 45 skipping gene mutation na DMD gene.
Nkwupụta: Amondys 45 bụ ọgwụ antisense oligonucleotide, antisense oligonucleotide nwere ike jikọta ya na ọnọdụ exon 45 nke pre-mRNA nke DMD gene, na-eme ka akụkụ nke exon 45 mechie mgbe emechara mRNA Excision tozuru etozu, si otú ahụ na-edozi akụkụ nke mRNA na-arụ ọrụ nke ọma, na-enyere aka mee ka ụfọdụ protein na-arụ ọrụ nke ọma, na-enyere aka mee ka ụfọdụ protein na-arụ ọrụ nke ọma. imeziwanye ihe mgbaàmà onye ọrịa.
(16) Amvuttra (vutrisiran)
Ụlọ ọrụ: Alnylam Pharmaceuticals mepụtara.
Oge ịre ahịa: FDA kwadoro maka ịre ahịa na June 2022.
Ngosipụta: Maka ọgwụgwọ transthyretin amyloidosis nke ketara eketa na polyneuropathy (hATTR-PN) na ndị okenye.
Amụma: Amvuttra (Vutrisiran) bụ ọgwụ siRNA na-ezubere transthyretin (ATTR) mRNA, nke a na-enye site na ntụtụ subcutaneous.Vutrisiran dabere na Alnylam's Enhanced Stability Chemistry (ESC) -GalNAc conjugate nnyefe ikpo okwu imewe na ụba ike na metabolic kwụsie ike.Nkwenye nke ọgwụgwọ ahụ dabere na data 9 nke ọnwa 9 nke usoro ọmụmụ ụlọ ọgwụ nke III (HELIOS-A), na n'ozuzu ya na-egosi na ọgwụgwọ ahụ mere ka mgbaàmà nke hATTR-PN dịkwuo mma, na ihe karịrị 50% nke ọnọdụ ndị ọrịa gbanwere ma ọ bụ kwụsị na-akawanye njọ.
4. Ọgwụ ọgwụgwọ mkpụrụ ndụ ndị ọzọ
(1) Rexin-G
Ụlọ ọrụ: Epeius Biotech mepụtara.
Oge ịzụ ahịa: Na 2005, Ụlọ Ọrụ Na-ahụ Maka Nri na Ọgwụ na Philippines (BFAD) kwadoro ya maka ịzụ ahịa.
Ngosipụta: Maka ọgwụgwọ ọrịa kansa dị elu na-eguzogide ọgwụ chemotherapy.
Okwu: Rexin-G bụ ogwugwu nanoparticle nke ejiri mkpụrụ ndụ ihe nketa.Ọ na-ewebata cyclin G1 mutant gene n'ime mkpụrụ ndụ ebumnuche site na vector retroviral iji gbuo etuto siri ike kpọmkwem.Usoro nchịkwa bụ infusion intravenous.Dị ka ọgwụ ezubere iche maka tumor nke na-arụsi ọrụ ike na-achọ ma na-ebibi mkpụrụ ndụ cancer metastatic, ọ na-enwe mmetụta ọgwụgwọ ụfọdụ n'ahụ ndị ọrịa dara ọgwụ ndị ọzọ nke ọrịa cancer, gụnyere ndị ezubere iche maka ihe ndị dị ndụ.
(2) Neovasculgen
Ụlọ ọrụ: Ụlọ ọrụ cell stem cell mepụtara.
Oge ndepụta: A kwadoro ya maka ịdebanye aha na Russia na December 7, 2011, wee malite na Ukraine na 2013.
Ngosipụta: Maka ọgwụgwọ nke ọrịa vaskụla akwara dị n'akụkụ, gụnyere ischemia siri ike.
Okwu: Neovasculgen bụ usoro ọgwụgwọ mkpụrụ ndụ ihe nketa dabere na plasmid DNA.A na-arụ mkpụrụ ndụ ihe nketa endothelial vaskụla (VEGF) 165 na ọkpụkpụ azụ plasmid ma tinye ya n'ime ndị ọrịa.
(3) Collategene
Ụlọ ọrụ: Ụlọ ọrụ mahadum Osaka jikọtara ọnụ na ụlọ ọrụ isi obodo.
Oge ịre ahịa: Ministri Ahụike, Ọrụ na ọdịmma nke Japan kwadoro na August 2019.
Ngosipụta: Ọgwụgwọ nke ischemia dị ala dị oke egwu.
Okwu: Collategene bụ usoro ọgwụgwọ mkpụrụ ndụ ihe nketa sitere na plasmid, ọgwụ izizi mkpụrụ ndụ ihe nketa ụlọ nke AnGes, ụlọ ọrụ na-ahụ maka ọgwụgwọ mkpụrụ ndụ ihe nketa na Japan mepụtara.Isi ihe dị na ọgwụ a bụ plasmid gba ọtọ nwere usoro mkpụrụ ndụ hepatocyte growth factor (HGF).Ọ bụrụ na a na-agbanye ọgwụ ahụ n'ime akwara nke akụkụ ahụ dị ala, HGF a gosipụtara ga-akwalite nhazi nke arịa ọbara ọhụrụ gburugburu arịa ọbara.Nnwale ụlọ ọgwụ ekwenyela na ọ na-emetụta ọnya ọnya.
Kedu ka Foregene nwere ike isi nyere aka mmepe ọgwụgwọ mkpụrụ ndụ ihe nketa?
Anyị na-enyere aka ịchekwa oge nyocha n'ọtụtụ nyocha, n'oge mmalite nke mmepe ọgwụ siRNA.
nleta nkọwa ndị ọzọ:
https://www.foreivd.com/cell-direct-rt-qpcr-kit-direct-rt-qpcr-series/
Oge nzipu: Dec-27-2022